Background: Increase of difficulty in drug development
Currently developing a new drug is estimated to cost more than $2Bn and take 13 years.
Possibility of success in drug development is one in millions shot. Under such circumstances, PDD based on gene expression profiles becomes more important to analyze drug efficacy to cells comprehensively.
Issue: Limit of existing drug discovery approach
Target-based drug discovery (TDD) approach, assessing whether drug candidates bind to target substance in human body, is commonly applied to drug discovery projects, but following issues would result in development cost increase:
- Few target substances for small molecule drugs remain in human body
- Drug candidates are prone to fail clinical trials because of low translatability of TDD
On the other hand, PDD had difficulty in assessment of phenotypes in a quantitative way and its throughput was also low because existing gene expression profiling technologies cost too much.
Solution 1: Our high-throughput whole transcriptome-based PDD
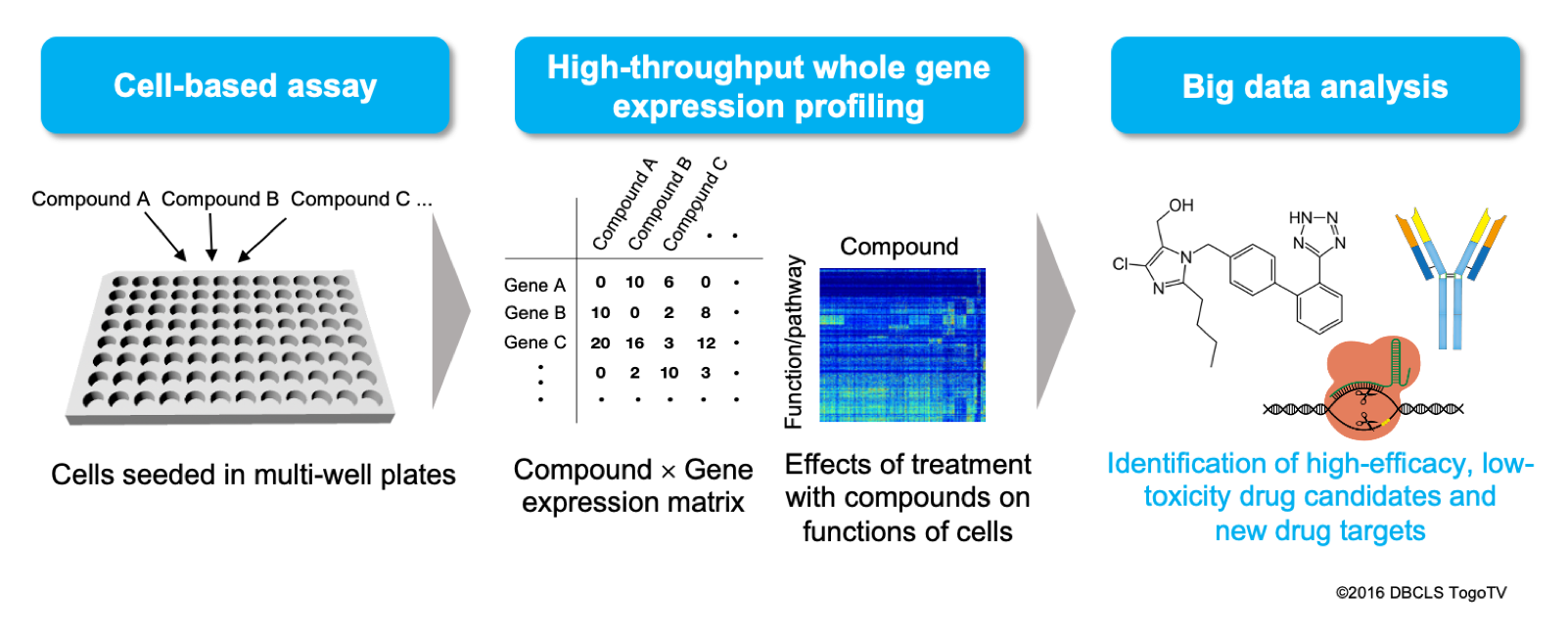
Based on our large-scale transcriptome technology, we can conduct high-throughput PDD by screening 10-100 times larger variety of compounds than ever. As whole gene expression profiles can detect influences of drugs on pathways in cells comprehensively, we can select compounds with novel mechanisms while evaluating drug efficacy and toxicity, as well as extrapolating drug target pathways and mechanisms.
Solution 2: Identification of stratification biomarkers
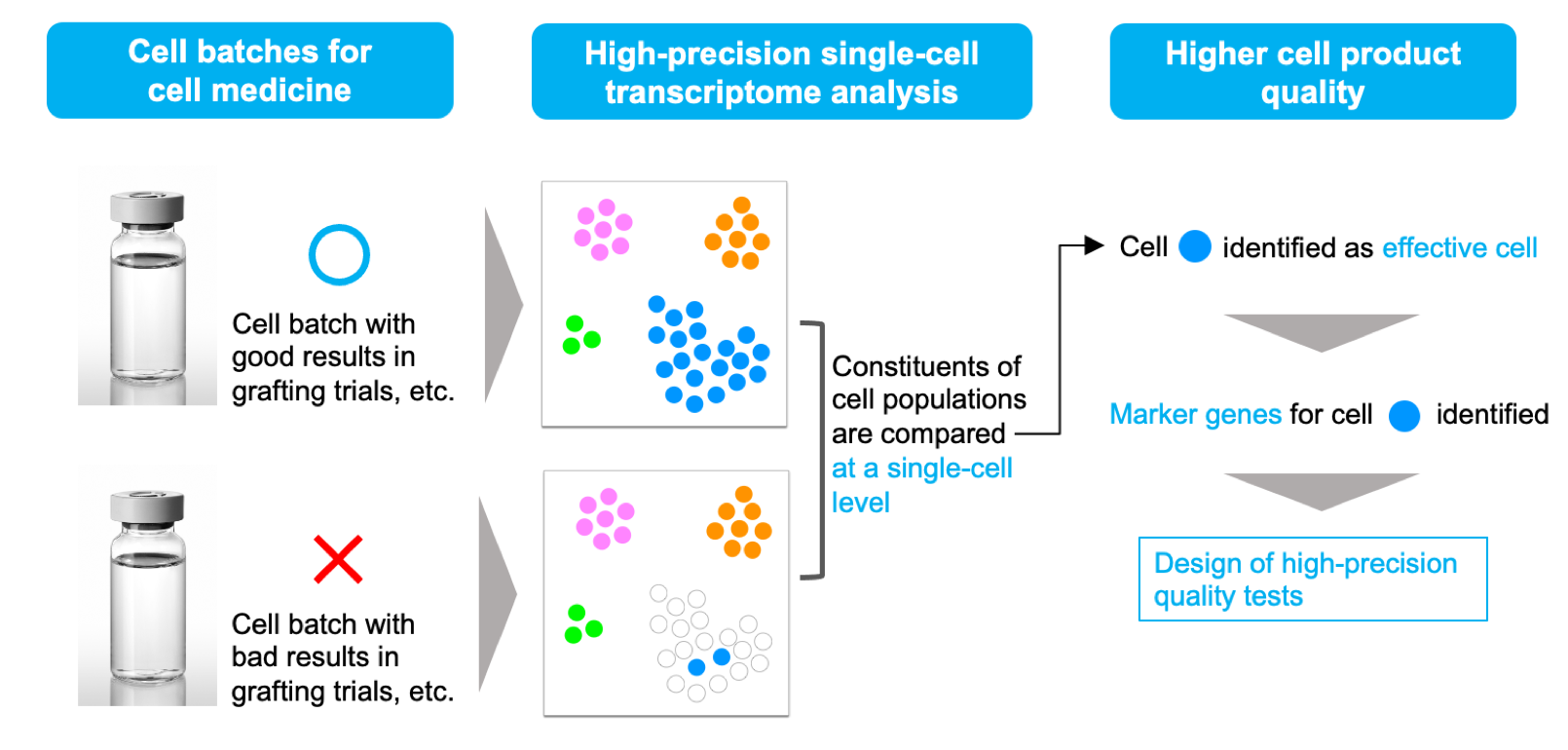
To increase the possibility of success in drug development, stratification biomarkers which distinguish successful patients who show drug effectiveness will be an important factor. We can identify stratification biomarkers by analyzing constituents of cell populations and gene expression profiles comprehensively based on our single-cell transcriptome technology with world’s best accuracy and artificial intelligence technology.
Background 1: Regenerative medicine is a dream medicine which cures illness with living cells
By transplanting living cells, regenerative medicine can regenerate organs and tissues which have lost their functions due to illnesses or injuries and restore them to their original states.
Regenerative medicine can be applied to a wider range of injuries and diseases, e.g. sequelae after cerebral infarction or spinal cord injury, chronic illnesses such as diabetes mellitus for which ongoing treatment imposes tremendous physical, time, and financial burdens, and disorders requiring organ transplant, etc. Thus, regenerative medicine offers an answer to a variety of unmet medical needs.
Background 2: All-out national commitment to development of regenerative medicine
Expectations surrounding regenerative medicine are extremely high. In Japan, collaborations between industry, academia, and government are enhanced today. An industry group comprising more than 250 participating companies, the Forum for Innovative Regenerative Medicine (FIRM), is at the center of industrial efforts to promote the widespread use of regenerative medicine, build a business ecosystem for industrialization, and make it internationally competitive. Academic bodies in Japan, particularly the Japan Society for Regenerative Medicine, are leading the world in regenerative medicine research and driving forward trials to translate the results of research into clinical applications. Politically, the Japan Agency for Medical Research and Development (AMED) has an enormous annual R&D budget of JPY15Bn to spend in the field. Recent regulatory developments also support prompt, safe treatment using regenerative medicine for the Japanese people. In Japan, major legislative measures including the Regenerative Medicine Promotion Act and the Therapeutic Products Act (the revised Pharmaceutical Affairs Act) were enacted in 2014. A rapid explosion in the regenerative medicine market is expected with current estimates of JPY12Tn and JPY38Tn globally (JPY1Tn and JPY2.5Tn domestically), for 2030 and 2050, respectively.
Issue: Variability in quality of cells
In order to manufacture cell-based products, cells must be cultured in a specific environment to the necessary amount for products. Because living cells have complex systems and they are sensitive to culture conditions (including culture media and scaffold), techniques, and process, they are prone to variability. How to overcome cell variability, which impacts the quality of cell-based products, is a major issue for regenerative medicine.
Solution 1: High-precision cell quality control
To date, it has been difficult to establish a quality test which can determine whether cell-based products have been correctly manufactured in high quality. By applying our single-cell transcriptome technology, it is possible to analyze compositions of target cells in cell-based products accurately, and identify cell quality marker genes which characterize the state of cells. This information can be utilized for establishment of high-precision quality tests, which realize stable manufacturing of high-quality cell medicine at a lower cost.
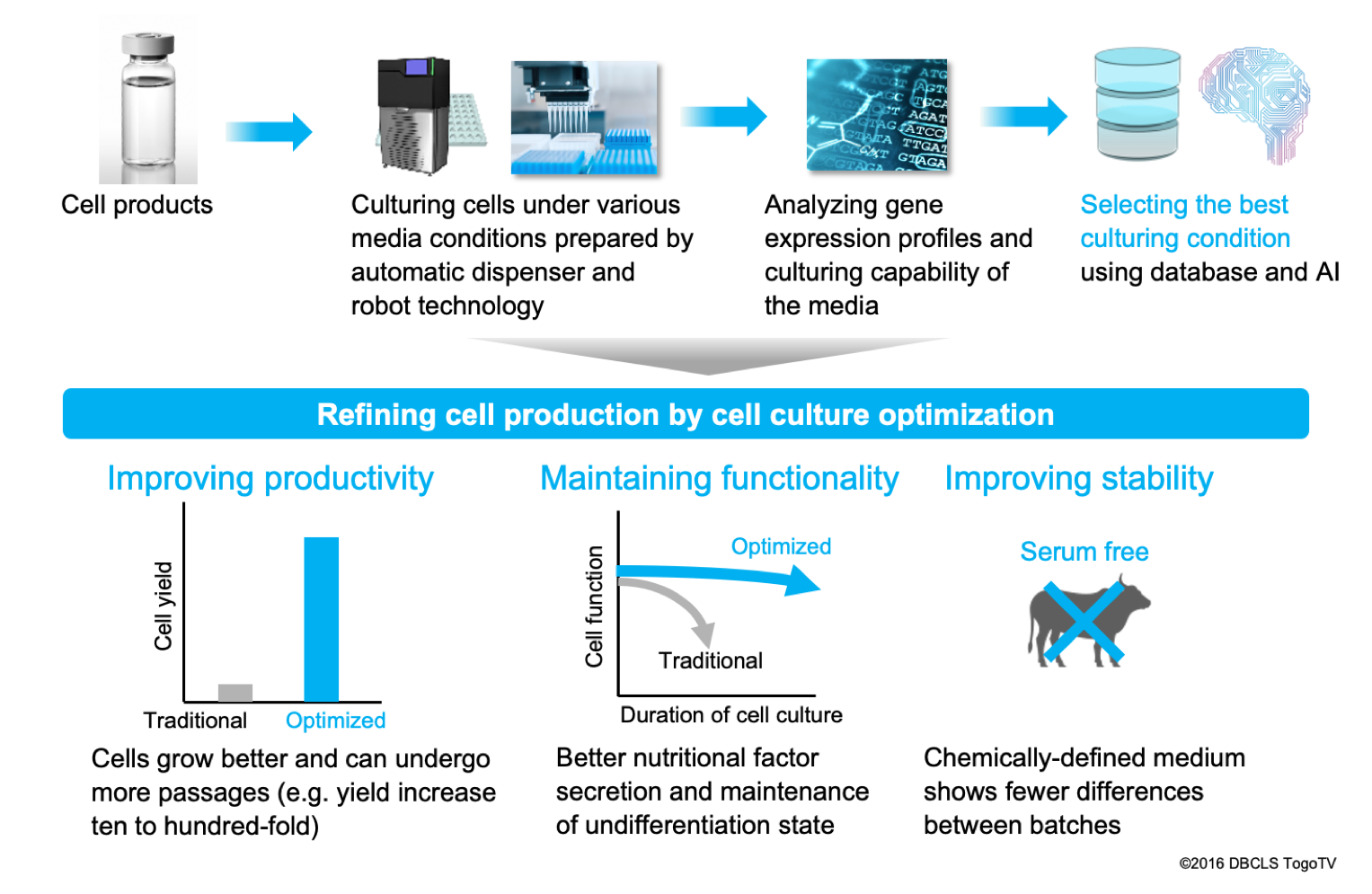
Solution 2: Optimization of cell culturing conditions
The second method for resolving variability in cell-based products is to fundamentally reduce the variability arising during manufacturing. As described above, if cell quality marker genes are identified, we can improve the cell culturing process more effectively. Based on solution chemistry and robotic technology, we prepare a large variety of culture conditions and conduct large-scale transcriptome analysis of the cells after culturing. By coupling the gene expression profiling data with information of the identified marker genes described above, we can then select the best culturing conditions for the cells. Under existing conditions, cells tend to lose their functional activity and proliferative ability through repeated passages. On the other hand, under the newly selected culture conditions, we can grow more cells for longer, and with greater stability while minimizing the difference between lots. Reduction of rejected lots and improvements in yield also help to cut manufacturing costs. We are now improving this technology toward completion.